1.1 Changes in physical state caused by the interaction between laser and materials The laser processing of metallic materials is mainly a thermal processing based on photothermal effects. When the laser irradiates the surface of the material, v...
Contact Us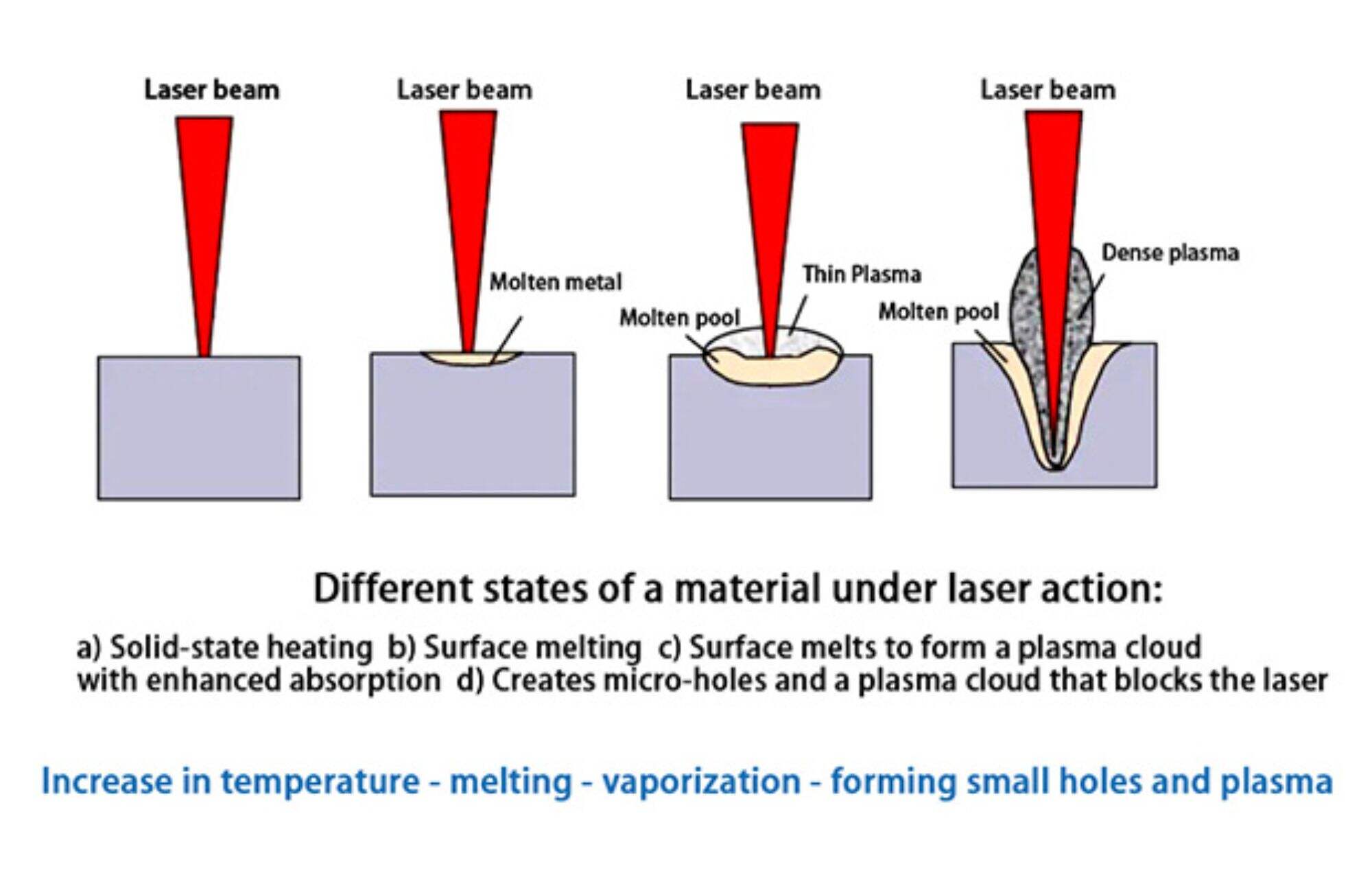
1.1 Changes in physical state caused by the interaction between laser and materials
The laser processing of metallic materials is mainly a thermal processing based on photothermal effects. When the laser irradiates the surface of the material, various different changes will occur in the surface area under different power densities.These changes include:
Melting: When a material absorbs laser energy, its temperature rises, possibly reaching its melting point, causing the material to transition from solid to liquid. This process is widely used in technologies such as laser welding, laser cladding, and laser rapid prototyping.
Evaporation and Sublimation: If the intensity of the laser is high enough to rapidly raise the material temperature above its boiling point, the material will transition directly from a solid or liquid state to a gaseous state. This process is used in technologies such as laser cutting, laser drilling, and laser evaporation.
Solidification: The process of material returning from a liquid state to a solid state after laser heating is called solidification. This process is common in the laser manufacturing process, especially in laser brazing and 3D printing technologies.
Annealing: By laser heating, the internal stress of the material can be redistributed, thereby achieving the purpose of reducing internal stress and improving material performance. This process does not accompany phase change, but it will cause the rearrangement of the crystal structure and changes in material properties.
Phase Transformation Hardening: Certain materials (such as steel) will undergo phase transitions during the cooling process, transitioning from a face-centered cubic structure (austenite) to a body-centered cubic structure (martensite). This transformation significantly enhances the hardness and strength of the material. Laser quenching utilizes this principle, by controlling the laser heating and cooling process, to achieve the hardening of the material surface or localized areas.
Photochemical Reaction: Laser irradiation can also trigger photochemical reactions in materials. These reactions include not only physical processes (such as photolysis, photo polymerisation) but also chemical processes, which can fundamentally change material properties. This principle is used in areas such as photolithography and material surface modification.
Photochromism: Some materials undergo photochromic reactions under laser irradiation, that is, a change in the color of the material. This change is caused by changes in the electronic structure of the material after absorbing light energy. This technology has potential application value in fields such as data storage and display technology.
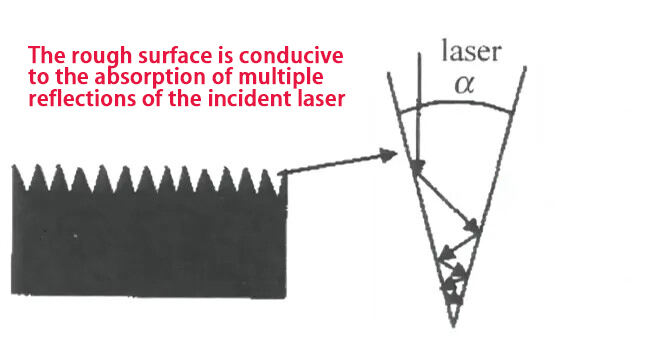
The relevant mechanism of action is shown in the figure below:
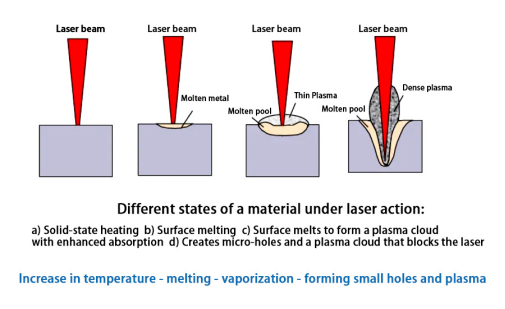
Under different conditions, when lasers with different wavelengths irradiate different metal materials, there will be certain differences in the specific values of the power density at each stage.In terms of material absorption of laser , the vaporization of the material is a dividing line. When the material does not vaporize, whether it is in the solid phase or the liquid phase, its absorption of laser only changes slowly with the increase in surface temperature; once the material vaporizes and forms plasma and keyhole, the material's absorption of laser, the absorption will suddenly change.
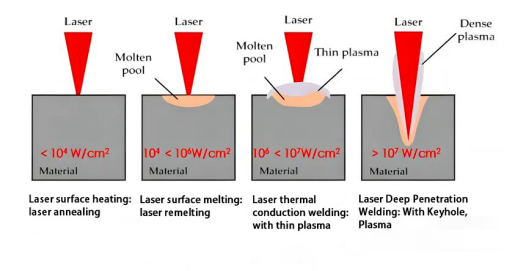
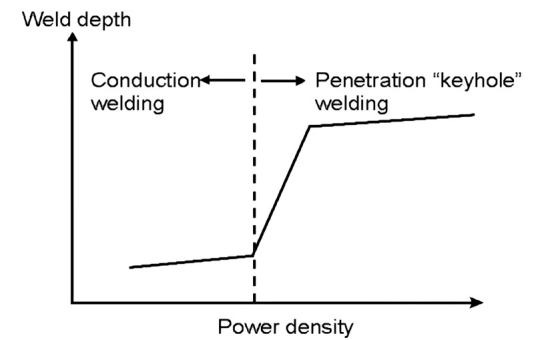
The figure below shows how the laser absorption rate of the material surface during laser welding changes with the laser power density and material surface temperature.When the material is not melted, the laser absorption rate of the material increases slowly as the surface temperature of the material increases. When the power density is greater than (10^6w/cm2), the material vaporizes violently, forming a keyhole, and the laser enters the keyhole and is reflected multiple times. Absorption causes the material's absorption rate of laser to increase dramatically, and the penetration depth will increase significantly.
1.2 Absorption of laser by metal materials—wavelength
Laser absorption mechanism:
The absorption of laser by metals is mainly achieved through the movement of free electrons. When a laser shines on the metal surface, its electromagnetic field will drive the free electrons in the metal to vibrate.This vibrational energy is then transferred in the form of heat to the metallic lattice structure, thereby heating the material. This absorption characteristic of metals makes them excellent materials for laser processing.
Effect of wavelength
Short wavelength (UV to visible light region):Metals generally absorb short wavelength laser more easily in the short wavelength range. This is because the free electrons in the metal can effectively interact with the electromagnetic field of short-wavelength light, causing energy to be transferred from the light wave into the metal, creating a thermal effect. Short-wavelength lasers can achieve higher positioning accuracy and smaller focus diameters, making them suitable for fine processing.
Mid-wavelength (near infrared region):Lasers in the near-infrared region, such as fiber lasers (wavelength approximately 1064 nanometers), have high absorption rates in metals and are the most commonly used wavelength range in metal processing.Laser of this wavelength can penetrate deep into the metal and has a relatively high absorption rate, making it suitable for deep processing and high-efficiency processing.
Long wavelength (far infrared region):For long-wavelength lasers, such as CO2 lasers (wavelength is about 10.6 microns), as the wavelength increases, the absorption efficiency of laser energy by metals usually decreases, which means that the reflection of long-wavelength lasers (such as far-infrared light) on the metal surface The rate is higher and the absorption rate is lower.The absorption rate in metals is relatively low. Nevertheless, because its wavelength is much larger than the free electron mean free path of metals, its absorption efficiency in some specific metallic materials is still acceptable. Long-wavelength lasers are primarily used for processing non-metallic materials, but they also have uses in some special metal processing applications.
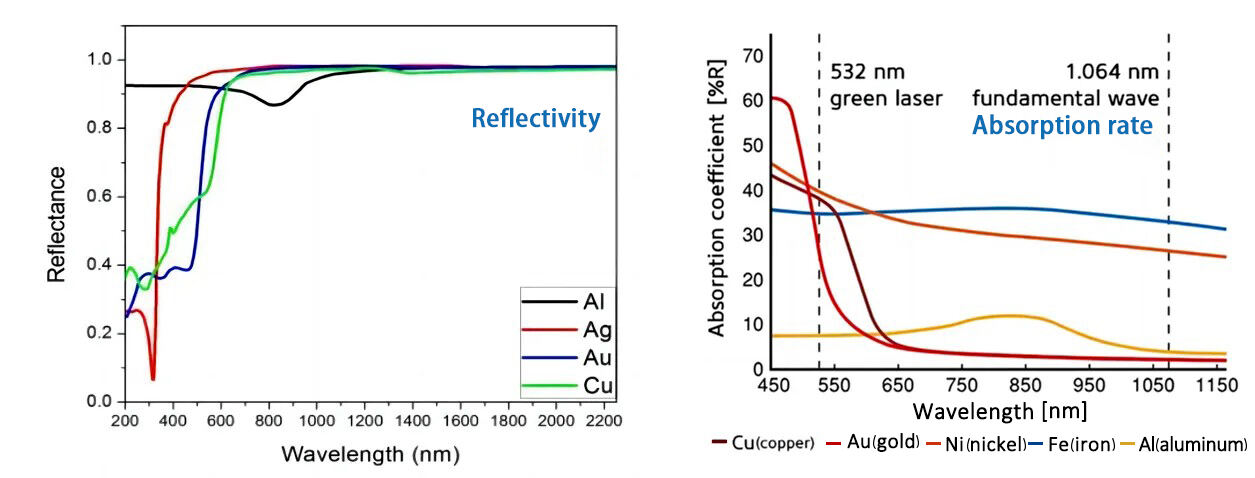
The figure below shows the relationship between reflectance, absorptivity and wavelength of commonly used metals at room temperature.In the infrared region, the absorptivity decreases and the reflectivity increases as the wavelength increases.Most metals strongly reflect 10.6um (CO2) wavelength infrared light, but have weak reflections on 1.06um (1060nm) wavelength infrared light. Metal materials have higher absorption rates for short-wavelength lasers, such as blue light and green light.
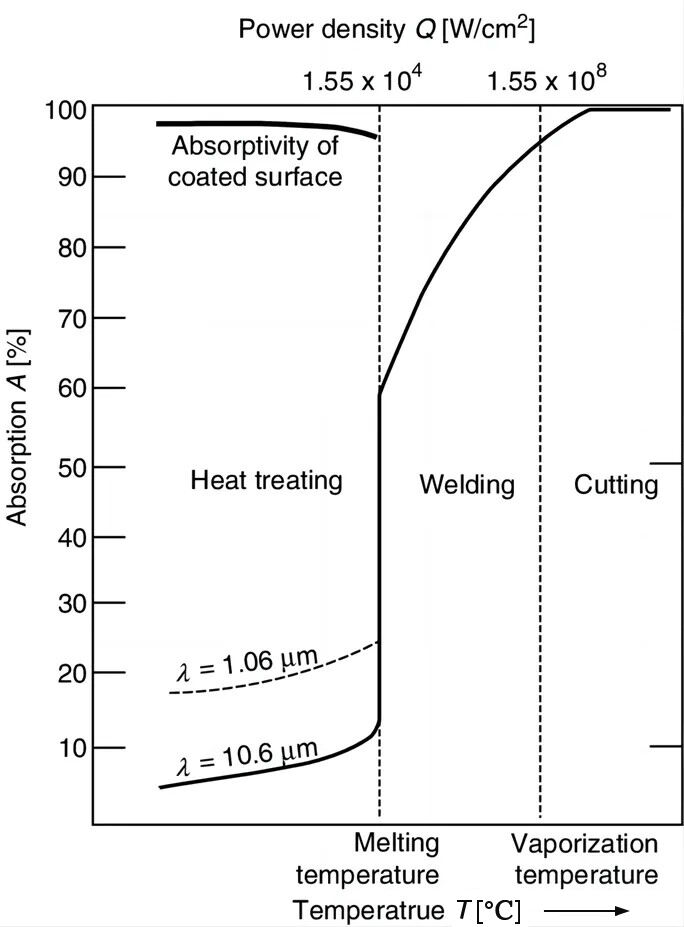
1.3 Laser absorption by metal materials—temperature
1.3.1 Absorption rates of different forms of aluminum alloys:
When the material is solid, the laser absorption rate is about 5-7%;
Liquid absorption rate to 25-35%;
It can reach more than 90% in the keyhole state.
1.3.2 The laser absorption rate of materials increases with temperature:
The absorption rates of metallic materials at room temperature are very small;
When the temperature rises close to the melting point, its absorption rate can reach 40%~60%;
If the temperature is close to the boiling point, the absorption rate is as high as 90%.
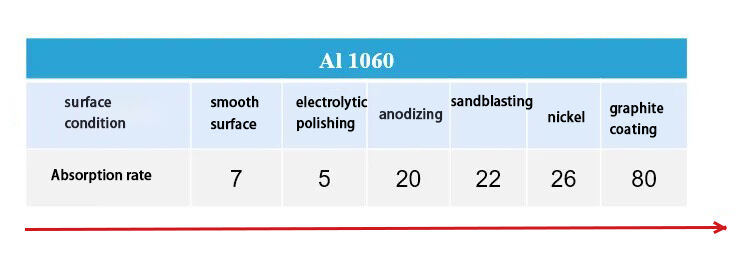
1.4 Laser absorption by metal materials—surface conditions
Conventional absorptivity is measured using a smooth metal surface. In practical applications of laser heating, it is usually necessary to increase the laser absorptivity of certain highly reflective materials (aluminum, copper) to avoid high reflection leading to false soldering;
The following methods can be used: Appropriate surface pretreatment processes are adopted to improve the reflectivity of laser. Prototype oxidation, sandblasting, laser cleaning, nickel plating, tin plating, graphite coating, etc. can all improve the laser absorption rate of the material.